此商品无价格,无法添加至购物车内,系统已自动发起询价,
询价单号 点击跳转查看 X
询价单号 点击跳转查看 X


Order NO. D291224
兔抗PRKCA/PRKCB/PRKCD/PRKCE/PRKCH/PRKCQ (phospho-Ser660)单克隆抗体
| 英文名 | Anti-PRKCA/PRKCB/PRKCD/PRKCE/PRKCH/PRKCQ (phospho-Ser660) rabbit monoclonal antibody | ||
| 别名 | protein kinase C alpha/beta/delta/epsilon/eta/theta; AAG6; PKCA; PRKACA; PKCI+/-; PKCalpha; PKC-alpha; PKCB; PRKCB1; PRKCB2; PKCI(2) | ||
| 相关类别 | 一抗 | 储存 | 冷冻(-20℃) |
| 宿主 | Rabbit | 反应种属 | Human, Rat |
| 标记物 | Unconjugate | 克隆类型 | rabbit monoclonal |
| 抗原 | PRKCA/PRKCB/PRKCD/PRKCE/PRKCH/PRKCQ (phospho-Ser660) | ||
| 编 号 | 包装 | 库存 | 目录价(¥) | 您的价格(¥) | 数 量 |
| D291224-0050 | 50 ul | 预计5日内发货 |
|
|
|
| D291224-0100 | 100 ul | 预计5日内发货 |
|
|
|
属性
| Background: | Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and the second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play a distinct role in cells. The protein encoded by the PRKCA gene is one of the PKC family members. This kinase has been reported to play roles in many different cellular processes, such as cell adhesion, cell transformation, cell cycle checkpoint, and cell volume control. Knockout studies in mice suggest that this kinase may be a fundamental regulator of cardiac contractility and Ca(2+) handling in myocytes. The protein encoded by the PRKCB gene is one of the PKC family members. This protein kinase has been reported to be involved in many different cellular functions, such as B cell activation, apoptosis induction, endothelial cell proliferation, and intestinal sugar absorption. Studies in mice also suggest that this kinase may also regulate neuronal functions and correlate fear-induced conflict behavior after stress. Alternatively spliced transcript variants encoding distinct isoforms have been reported. The protein encoded by the PRKCD gene is a member of the protein kinase C family of serine- and threonine-specific protein kinases. The encoded protein is activated by diacylglycerol and is both a tumor suppressor and a positive regulator of cell cycle progression. Also, this protein can positively or negatively regulate apoptosis. Defects in this gene are a cause of autoimmune lymphoproliferative syndrome. The protein encoded by the PRKCE gene is one of the PKC family members. This kinase has been shown to be involved in many different cellular functions, such as neuron channel activation, apoptosis, cardioprotection from ischemia, heat shock response, as well as insulin exocytosis. Knockout studies in mice suggest that this kinase is important for lipopolysaccharide (LPS)-mediated signaling in activated macrophages and may also play a role in controlling anxiety-like behavior. The protein encoded by the PRKCH gene is one of the PKC family members. It is a calcium-independent and phospholipids-dependent protein kinase. It is predominantly expressed in epithelial tissues and has been shown to reside specifically in the cell nucleus. This protein kinase can regulate keratinocyte differentiation by activating the MAP kinase MAPK13 (p38delta)-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha (CEBPA). It is also found to mediate the transcription activation of the transglutaminase 1 (TGM1) gene. Mutations in this gene are associated with susceptibility to cerebral infarction. The protein encoded by the PRKCQ gene is one of the PKC family members. It is a calcium-independent and phospholipid-dependent protein kinase. This kinase is important for T-cell activation. It is required for the activation of the transcription factors NF-kappaB and AP-1, and may link the T cell receptor (TCR) signaling complex to the activation of the transcription factors. |
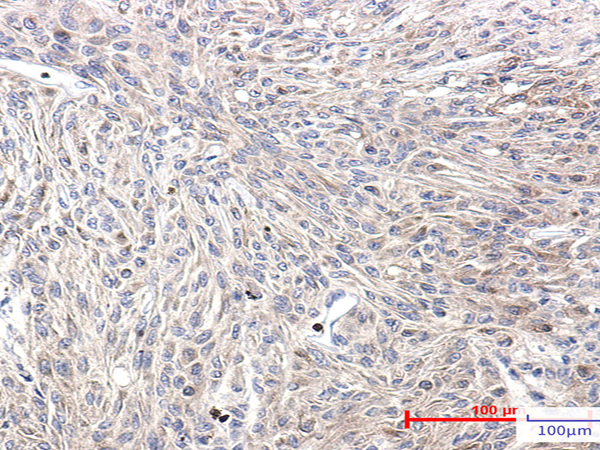
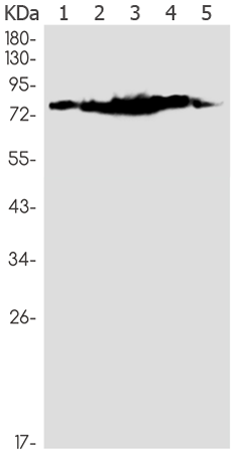
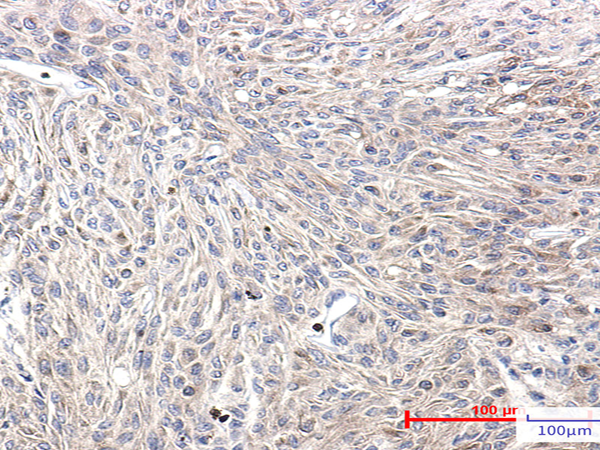
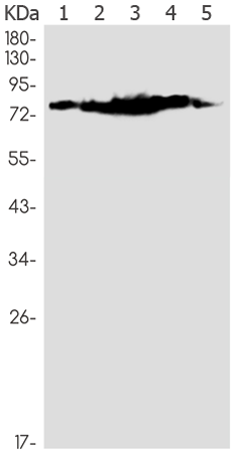
| Applications: | WB, IHC |
| Name of antibody: | PRKCA/PRKCB/PRKCD/PRKCE/PRKCH/PRKCQ (phospho-Ser660) |
| Immunogen: | Synthetic phosphopeptide corresponding to residues surrounding Ser660 of human PRKCA |
| Full name: | protein kinase C alpha/beta/delta/epsilon/eta/theta |
| Synonyms: | AAG6; PKCA; PRKACA; PKCI+/-; PKCalpha; PKC-alpha; PKCB; PRKCB1; PRKCB2; PKCI(2); PKCbeta; PKC-beta; MAY1; PKCD; ALPS3; CVID9; nPKC-delta; PKCE; nPKC-epsilon; PKCL; PKC-L; PRKCL; uORF2; nPKC-eta; PRKCT; nPKC-theta |
| SwissProt: | P17252/P05771/Q05655/Q02156/P24723/Q04759 |
| IHC positive control: | Human Brain |
| IHC Recommend dilution: | 50-100 |
| WB Predicted band size: | 77 kDa |
| WB Positive control: | Hela, A549, HL-60, U2OS, C6 lysates |
| WB Recommended dilution: | 500-2000 |






















 精确搜索
精确搜索 我的草稿
我的草稿
 我的购物车
我的购物车

 精确搜索
精确搜索